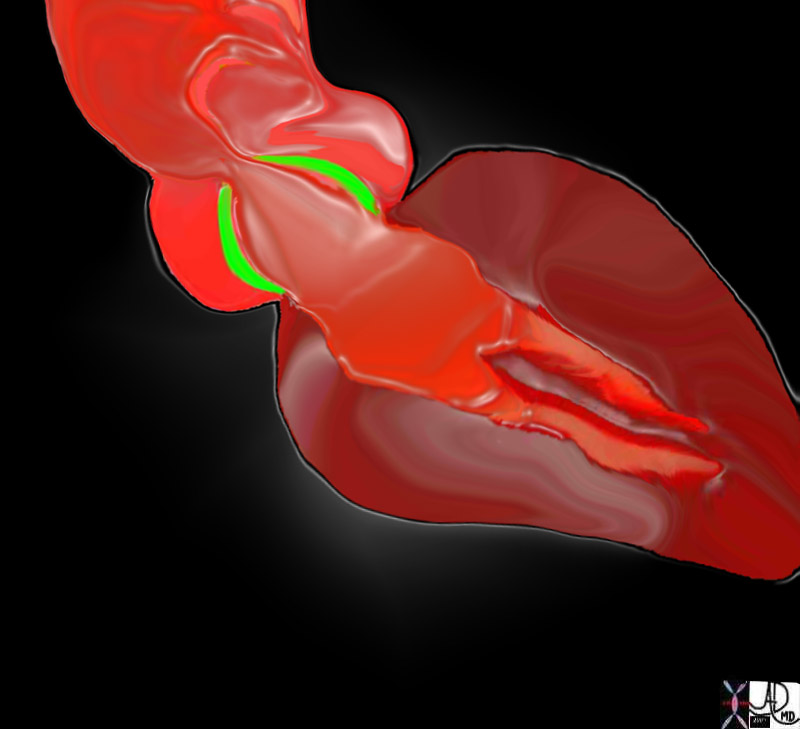
Aortic Stenosis
Copyright 2008
Definition
Ashley Davidoff TheCommonVein.net
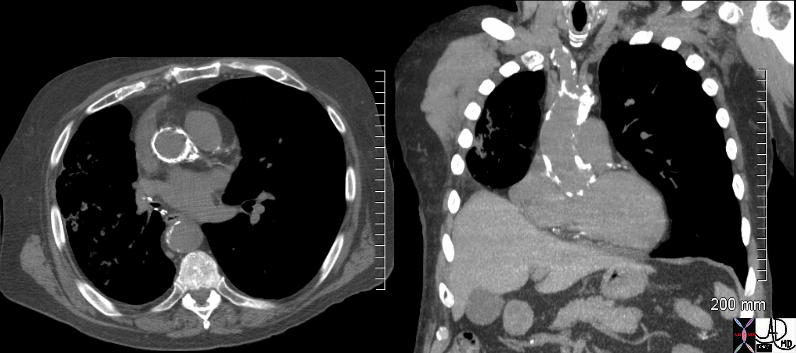
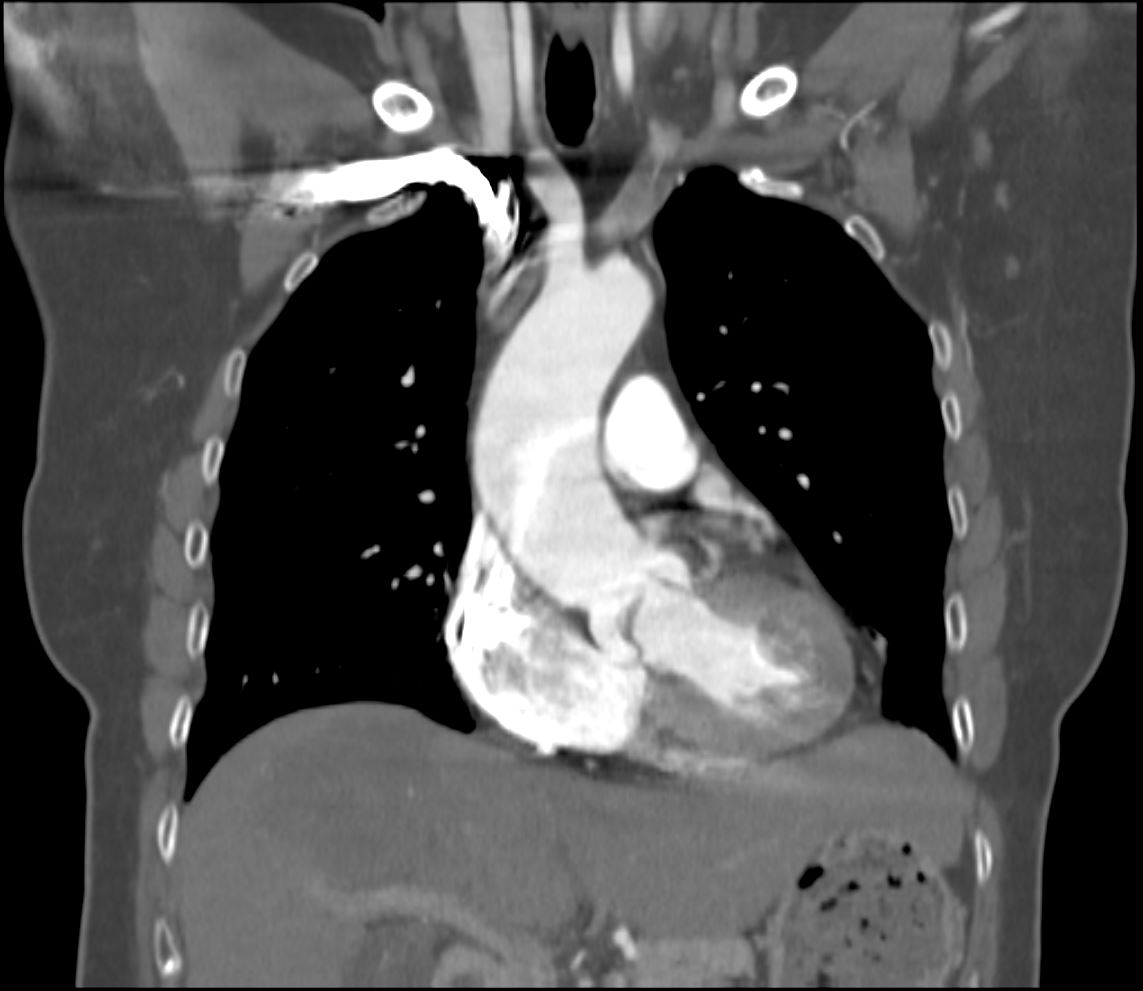
CT in the sagittal plane in a 79year old male shows calcification of the aortic valve along the commissures (magnified in image below) indicating mild to moderate aortic stenosis
Ashley Davidoff MD TheCommonVein.net 136392c
Aortic stenosis (AS) is a mechanical disorder of the heart, and is the most common valvular abnormality. It is most commonly caused by age related degenerative and calcific changes in adults, while congenital bicuspid aortic valve is the most common congenital abnormality in the pediatric population. The narrowing of the aortic valve results in a progressively increasing pressure gradient between the left ventricle (LV) and the aorta. Compensatory increase in LV mass and concentric LV hypertrophy follows which may eventually be complicated by heart failure or arrhythmias late in the disease.
GA Aortic stenosis (AS) , is the obstruction of blood flow across the aortic valve. AS has several causes: congenital unicuspid or bicuspid valve, rheumatic fever, and degenerative calcific changes of the valve. Structurally the aortic valve becomes stenotic and functionally there is a resistance to systolic ejection resulting in a systolic pressure gradient between the left ventricle and the aorta , in aortic stenosis . Clinically , a harsh ejection systolic murmur radiating to the carotids can be appreciated in the aortic area in patients with stenotic aortic valve . The Two-dimensional transthoracic echocardiography can confirm the clinical diagnosis of AS . Using echo-Doppler techniques, the systolic pressure gradient across the aortic valve can be assessed. Medical or surgical therapies may be used for treatment depending on the severity .
Clinically aortic stenosis is frequently asymptomatic and should be suspected in patients presenting with exertional dyspnea, angina pectoris, and syncope. The diagnosis is confirmed by a combination of physical findings including an ejection systolic murmur and 2D Echocardiography that enables an estimate of the gradient, and the differentiation between a degenerated valve and bicuspid valve.
Treatment is surgical and requires aortic valve replacement.
Besides valvular lesions, supravalvular (narrowing of the ascending aorta or by a fibrous diaphragm) and subvalvular lesions (hypertrophic cardiomyopathy or membranous diaphragm or a fibrous ridge just below the aortic valve) can also cause aortic stenosis.
Principles
Structural Principles
The aortic valve is a semilunar (half moon shaped) tricuspid valve (3 cusps) located at the junction of the LV outflow tract and ascending aorta that enables one way flow of blood. The three cusps are the right cusp located in the anterior wall, left and posterior cusps (non coronary cusp) located in the posterior wall. Dilatation of the wall of the aorta behind these cusps is called the aortic sinuses of Valsalva.
The normal open aortic valve is 3-4 cm2. A decrease in the size of this opening is the key principle in the disease process.
Physiological and Pathophysiological Principles
The output of the heart and hence the transport of blood, depends not only on the strength of the pump, but also on normally sized valves both at the inflow and outflow level.
During normal ventricular systole isovolumetric (isovolumic, isometric) ventricular contraction lasts about 0.05 s, until the pressures in the left and right ventricles exceed the pressures in the aorta (80 mm Hg) and pulmonary artery (10 mm Hg) and the aortic and pulmonary valves open. Normally there is a very small difference in pressure gradient between the LV and aorta .
In aortic stenosis there is an increase in isovolumetric contraction time to generate the increased systolic pressure in the LV so as to overcome the obstruction to LV out flow.
The obstruction to LV outflow produces
1. An increase in afterload and a systolic pressure gradient between the LV and aorta .
2. Compensatory increase in LV mass by concentric hypertrophy which helps to preserve the LV systolic function until late in the disease.
3. LV diastolic dysfunction – as seen by an elevated LVEDP(left ventricular end diastolic pressure)secondary to chamber stiffness causing impaired LV relaxation , decreased LV compliance by an increased after load and relative myocardial ischemia of the subendocardial region.
Late in the course of the disease cardiac output, stroke volume, and LV and aorta pressure gradient decrease, while mean left atrial pressure, PCWP, and right sided pressures rise and herald the development of systolic LV dysfunction.
Severe aortic stenosis is defined by a peak systolic pressure gradient >50 mmHg in the face of a normal cardiac output or an effective aortic orifice less than approximately 1.0 cm2 or <0.6 cm2/m2 body surface area (<1/3rd of normal).
A large a wave is seen on the left atrial waveform in severe aortic stenosis because of a combination of vigorous contraction of a hypertrophied LA and reduced LV compliance. This atrial kick is essential to increase LV volume and raises LVEDP to ensure effective contraction and also to decreases pulmonary venous and capillary pressures from rising, which otherwise would result in pulmonary congestion. Loss of appropriately timed atrial contraction with atrial fibrillation or AV dissociation results in a rapid downhill course. The CO at rest is within normal limits in most patients with severe AS, but it usually fails to rise normally during exercise. Other potential complications include arrhythmias, atrial fibrillation, infective endocarditis, embolic stroke, sudden cardiac death, GI bleeding with angiodysplasia.
The heart compensates by increasing LV mass, increasing LV systolic pressure, and prolonging the systolic ejection phase. The increased LV mass results in a relative decline in myocardial capillary density. Additionally there is a reduced diastolic transmyocardial (coronary) perfusion gradient due to elevated LVEDP. All these factors decrease oxygen supply. In general the left ventricle can tolerate the increased pressure requirements since by design it is the “pressure ventricle”. Thus symptoms of angina and congestive heart failure are usually relatively late complications, and are caused by relative ischemia in the subendocardium.
Exertional syncope is caused by vasodilatation in exercising muscles and inadequate vasoconstriction in non exercising ones in the face of a fixed cardiac output. Rarely arrhythmias may occur and are usually ventricular in origin.
Dyspnea is secondary to elevated LVEDP which causes a rise in pulmonary capillary pressure and leakage of fluid into the interstitium thickening of the alveolar membrane and a less efficient oxygen exchange mechanism.
Principles of Disease
Aortic Stenosis is a mechanical disorder, and is basically a narrowing of one of the tubular systems of the body at the level of the valve. As a mechanical disorder it is treated with surgery which in general, is the approach to therapy when mechanical disorders such as obstructions and ruptures occur. The narrowing may be caused as stated above by the bicuspid valve which may be narrow from birth, or which becomes narrowed with wear and tear. Aortic sclerosis is a degenerative condition of the aorta seen in the older population where dystrophic calcification of the degenerating aortic valve and annulus causes progressive narrowing. Rheumatic aortic stenosis used to be far more common in both the pediatric and adult population, but is relatively rare in Western cultures but still found in developing cultures. In this instance immunoinflammatory response to a specific streptococcus, results in fibrosis and calcification of the valve.
Cause
The disease is often caused by a congenital bicuspid valve as a result of fusion of the commissures. This entitiy is seen in 1-2% of population, with a dominance in males and M>F; commonly seen in association with coarctation of the aorta. Hypoplastic valve is also a cause of aortic stenosis. Acquired causes include aortic sclerosis and rheumatic heart disease.
Bicuspid Aortic Valve (BAV)
Bicuspid aortic valve is one of the most common congenital lesions of the cardiovascular system. In this entity only two aortic valve cusps are developed.
Abnormal architecture of leaflets produced by developmental commissural fusion, renders them susceptible abnormal structural changes to ordinary hemodynamic stresses, which ultimately lead to valvular thickening, calcification, increased rigidity, and narrowing of the aortic orifice.
Patients with BAV commonly have aortic narrowing and regurgitation of variable severity. Most are usually isolated mild lesions but are prone to progressive calcification, and advancing aortic stenosis later in life. With time the gradient increases and the left ventricle hypertrophies to accommodate the increased pressure and work load. The structural distortion of the valve predisposes it to infection and BAV therefore can be complicated by bacterial endocarditis.
Heart murmurs result from either regurgitation or stenosis and are best diagnosed by echocardiography
Surgery with replacement of the valve is indicated when the pressure gradient become high, usually with gradients that are greater than 50-60mmHg across the valve..
|
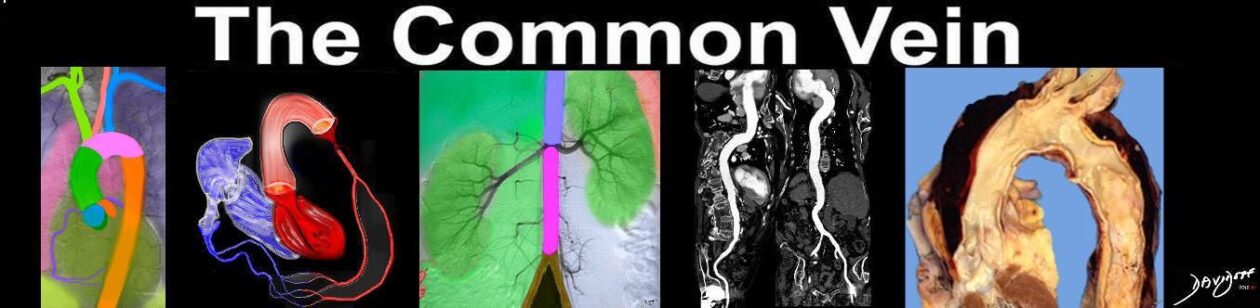
Bicuspid Aortic Valve Caused by Fusion of Two Commissures Between the Right and Non Coronary Cusp |
| 07951b Davidoff MD |
|
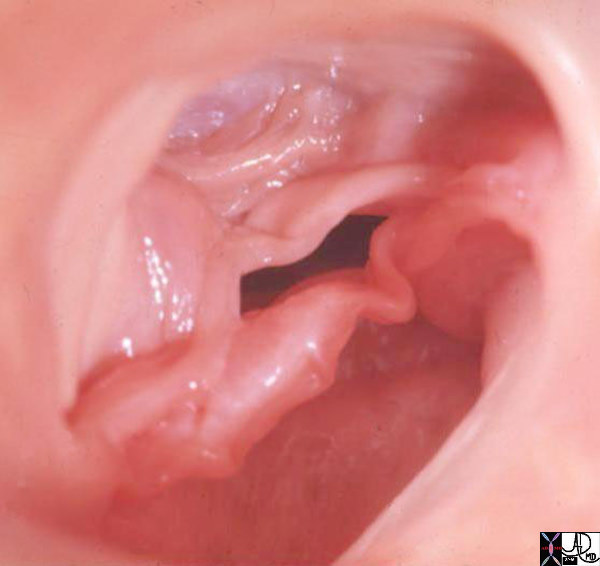
Normal and Thickened Aortic Valve over Time |
| 07953c02 heart cardiac aorta aortic valve fx normal fx thickened fx bicuspid aortic valve fx calcified fx calcification fusion of the intercoronary commisures grossanatomy grosspathology Davidoff MD b Courtesy Henri Cuenoid MD |
Aortic Sclerosis
Aortic sclerosis is a degenerative disease commonly seen in older patients and caused by wear and tear on the valve as well as an atherosclerotic process. Calcium deposition due to processes similar to atherosclerotic vascular disease is responsible for degenerative aortic valve disease. Histological examination of degenerative valves exhibits inflammatory changes, as seen in atherosclerotic vessels and atherosclerotic risk factors, such as age, male sex, smoking, diabetes mellitus, hypertension, increased LDL, reduced HDL cholesterol, and elevated C-reactive protein predispose to aortic valve calcification.
It results in the progressive calcification of the aortic annulus and/or aortic valve. This disease also induces thickening of the arterial wall. These effects cause heightened pressures in the left ventricle, increasing the risk of heart attack. The aortic annulus is prone to calcification because fatty acid deposits easily accumulate on the aortic surface in juncture with the valves. The diagnosis of the disease is based on the detection of a calcified annulus or valve by CXR or CTscan while the diagnostic procedure of choice is conventional or transthoracic echocardiography which not only confirms the diagnosis but also assesses the severity of the gradient. If severe, surgical treatment is required.
|
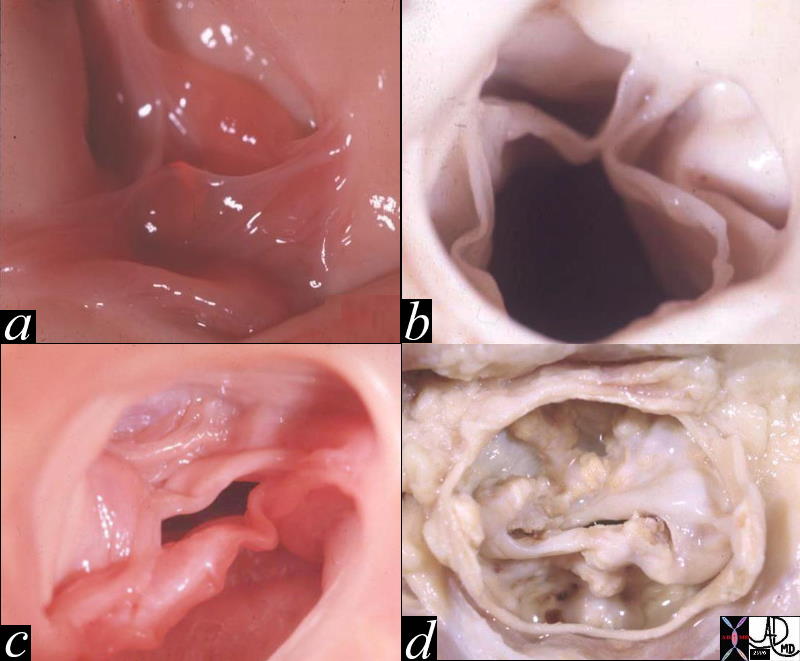
Calcified Aortic Annulus |
| 72864.801c01 72864.800 aorta aortic annulus fx calcified calcification dx aortic sclerosis CXR plain film of chest Courtesy Ashley DAvidoff MD |
|
Calcified Annulus and Calcified Atherosclerotic Ascending Aorta |
| 72859c01 aorta aortic annulus fx calcified calcification dx aortic sclerosis CTscan Courtesy Ashley Davidoff MD |
Diagnosis
Clinical
Clinically the patient presents with a systolic murmur and a plateau pulse, with signs of hypertrophy of the left ventricle.
Symptoms are likely once the peak echo gradient is > 50 mm Hg
Stroke volume declines, the systolic pressure falls and the pulse pressure narrows.
Peripheral arterial pulse palpated in the carotid or brachial arteries, rises slowly to a delayed sustained peak (pulsus parvus et tardus) not seen in elderly due to stiffening of the arteries.
a wave in the jugular venous pulse is accentuated by diminished distensibility of the RV cavity caused by the bulging, hypertrophied intraventricular septum.
Systolic thrill is generally present at the base of the heart, in the jugular notch, and along the carotid arteries.
Auscultation
The intensity of the systolic murmur does not correspond to the severity of AS, rather, the timing of the peak and the length or duration of the murmur corresponds to the severity of AS. The more severe the stenosis, the longer the duration of the murmur and the more likely it peaks at mid-to-late systole.
S1 usually is normal or soft. The aortic component of the second heart sound, A2, usually is diminished or absent because the AV is calcified and immobile and/or aortic ejection is prolonged or buried in the prolonged systolic ejection murmur. Paradoxical splitting of the S2 also occurs because of late closure of A2; its absence usually excludes severe AS.
A prominent S4 usually is present due to forceful atrial contraction
EKG
LV hypertrophy and repolarization changes like ST segment depression and T wave inversion are seen on standard leads I and aVL and in the left precordial leads.
Imaging
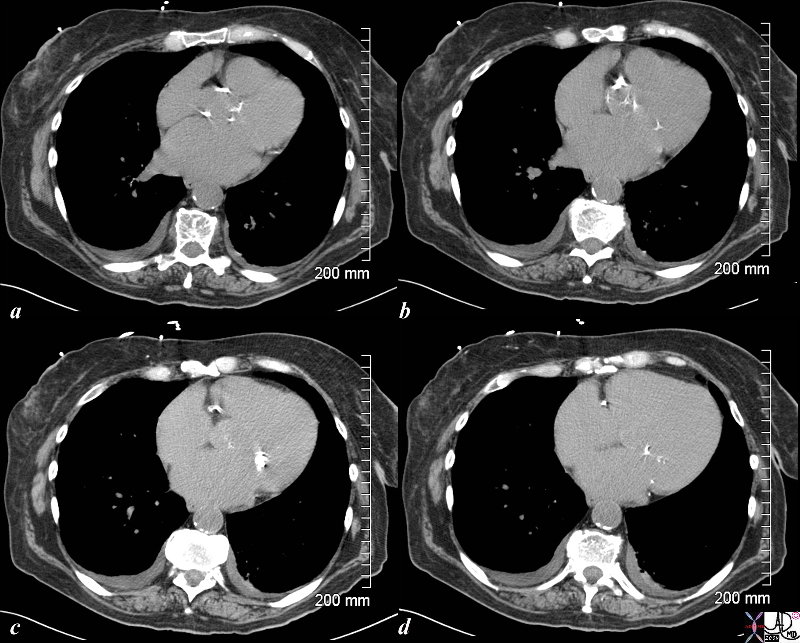
CXR shows left ventricular configuration, ascending ectasia, projection. Its presence on the plain film suggests significant stenosis, usually more than 50 mmHg. CT is more sensitive to the calcification and is more commonly not assocaited with a gradient. Gated CT can be used to quantify LVH and ejection fraction.
Echocardiography
M- Mode LV ejection time , aortic root diameter and LA diameter can be measured . 2D Echo – parasternal long and short axis views and apical 5chamber view. – Thickened cusps , Reduced motion -Doming LVH concentric Post stenotic dilatation may be seen
Evaluation of leaflet motion as maximal aortic cusp separation (MACS). When MACS is <11mm, stenosis may be inferred as severe and valve surface area is ~.75cm2. Then MACS is >13mm, stenosis is considered to be mild with aortic valve surface area ~>1cm2
Doppler Echo Severity of AS by estimating 1.pressure gradient (mean and peak )across the AV and 2.valve area continuity equation.
Major limitation of Doppler echocardiography in estimating the severity of AS is
Underestimation of the gradient if the beam is not parallel to the AS velocity jet. Thus, in a patient with clinical features of severe AS but echo/Doppler findings of mild-to-moderate AS, further evaluation with repeat Doppler or catheterization is required.
Very rarely, Doppler may overestimate the mean gradient in cases of severe anemia (hemoglobin <8 g/dL), a small aortic root, or sequential stenoses in parallel (coexistent LVOT and valvular obstruction)
|
Aortic Stenosis |
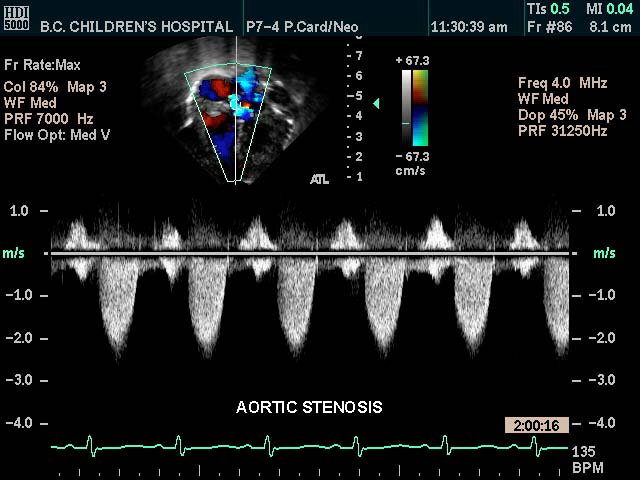
| This color flow doppler echo of the heart with pulse flow interrogation at the aortic orifice showing a short-axis aorta left atrial view, and demonstrating high velocity The patient has a diagnosis of aortic stenosis
Courtesy Philips Medical Systems 33144 code cardiac heart echo aorta AOV AV AS imaging cardiac echo tube principle |
|
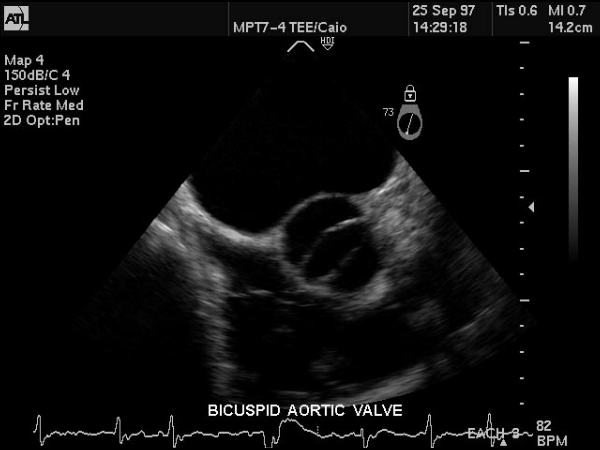
Bicuspid Aortic Valve |
| This gray scale echo of the heart showing a short-axis aorta left atrial view, and demonstrating the aortic valve with two cusps. The patient has a diagnosis of bicuspid aortis valve which is a congenital condition. Courtesy Philips Medical Systems 33169 code cardiac heart echo aorta bicuspid aortic valve congenital imaging cardiac echo |
Echocardiographic severity
|
Indicators |
Mild |
Moderate |
Severe |
Critical |
|
Jet velocity m/sec |
<3 |
3.0-4.0 |
>4 |
|
|
Mean gradient mm Hg |
<25 |
25-40 |
>40 |
>80 |
|
Valve area cm2 |
>1.5 |
1.0-1.5 |
<1 |
<.7 |
|
Valve area index cm2/m2 |
|
|
<.6 |
|
|
Aortic Stenosis – Turbulence Shown by MRI |
|
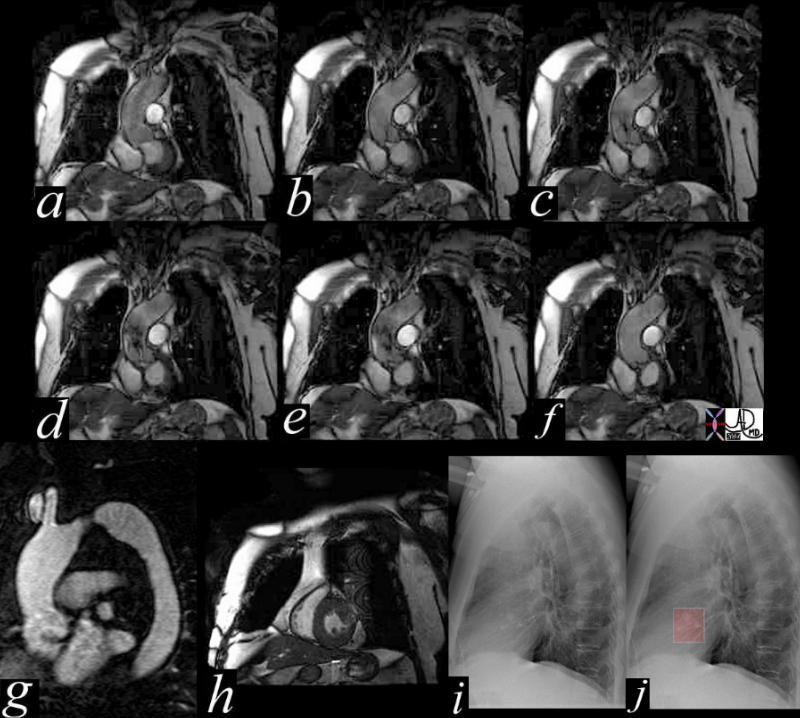
This series of coronal MRI images of the aortic valve (a-f) show phases from diastole (a) through systole (b,c,d,e) with a narrow (b,c) and then turbulent jet, (d,e) back to diastole (f) Image g shows a thickened valve, while the short axis of the LV (h) shows LV hypertrophy. The plain film of h and i highlight the calcific nature of the valve. The diagnosis is aortic valve stenosis. Courtesy Scott TSai MD 38871c01 code cardiac heart aortic valve AS LVH calcification calcified imaging radiology MRI CXR plain film |
Cardiac Catheterization
Catheterization of the left side of the heart and coronary arteriography should be carried out in patients suspected of having severe AS, who are being considered for operative treatment.
1.Patients with clinical signs of AS and symptoms of myocardial ischemia, in whom associated coronary artery disease is suspected.
2. Patients with multivalvular disease.
3.Patients in whom a sub- or supravalvular obstruction to LV outflow is suspected.
|
Aortic Stenosis – Doming of the Valve – Pliable Valve |
| 07969bW.802 heart cardiac aorta aortic valve fx thickening of the aortic valves LVH left ventricular hypertrophy post stenotic dilatation of the ascending aorta turbulence eccentric jet doming of the aortic valve AV AS aortic stenosis Davidoff art |
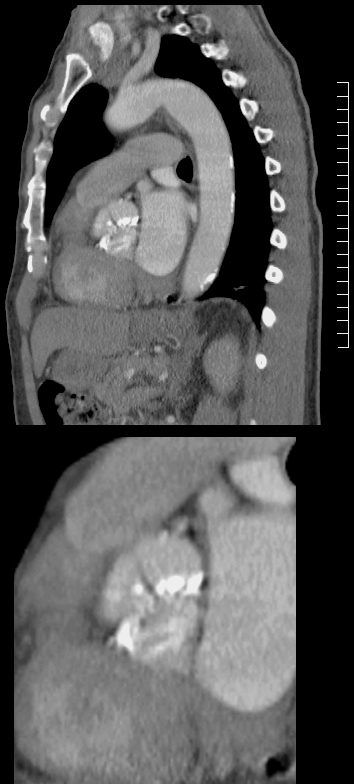
76655c02.8s |
| 76655c02.8s heart cardiac mitral valve MV MS mitral stenosis calcification calcified valve aorta aortic valve aortic calcifications CTscan Courtesy Ashley Davidoff MD copyright 2009 all rights reserved |
Prognosis
Obstructive calcific AS is a progressive disease, with an annual reduction in valve area of approximately 0.1 mm2/year.The average time to death after the onset of angina pectoris is 3 years; syncope is 3 years; dyspnea 2 years; and congestive heart failure, 1.5 to 2 years. Congestive heart failure is the cause in two thirds. Sudden death is rare.
Treatment
The need for aortic valve replacement is based on severity of the disease. Assessment of the coronary arteries prior to surgery is essential, in case CABG is warranted.
Asymptomatic individuals
Frequent monitoring for development of symptoms and disease progression.Serial echocardiograms , severe AS – an echocardiogram annually, moderate AS, serial every 1 to 2 years, and in patients with mild AS, every 3 to 5 years. More frequent Echocardiograms if there is a change in signs or symptoms.
2.Rheumatic prophylaxis and Endocarditis prophylaxis .
Symptomatic Individuals
Aortic valve replacement or percutaneous valvuloplasty . There is no role for medical management except as a bridge to operative management.
Indications for operative management
Aortic valve replacement or percutaneous valvuloplasty . There is no role for medical management except as a bridge to operative management.
Indications for operative management
1.Patients with severe AS (valve area <1.0 cm2 or 0.6 cm2/m2 body surface area) who are symptomatic,
2. those who exhibit LV dysfunction EF<50,
3. expanding poststenotic aortic root, even if they are asymptomatic.